Breathing Room: How Covalent Organic Frameworks Could Rescue Metal-Gas Batteries
Source PublicationChemical Society Reviews
Primary AuthorsWang, Yan, Peng et al.
The promise is seductive. Imagine a battery that breathes, drinking in oxygen or carbon dioxide from the air to generate power, offering energy densities that dwarf our current lithium-ion standards. It sounds like magic. But in practice, the metal-gas battery is a tragedy of suffocation. Inside these cells, a silent war of attrition rages. The chemical reactions are agonizingly slow, a lethargic drift rather than a snap of power. Worse is the clogging. As the battery discharges, solid byproducts build up like arterial plaque, choking the electrodes in a process known as passivation. The electrolyte, the very lifeblood of the system, often decomposes, unable to withstand the harsh chemical environment. For years, scientists have watched these devices fail, not for lack of potential, but because the internal architecture collapses under its own ambition. The dream of a lightweight, high-capacity energy source remains trapped behind these walls of inefficiency and instability. The physics seemed unforgiving, and the chemistry, insurmountable.
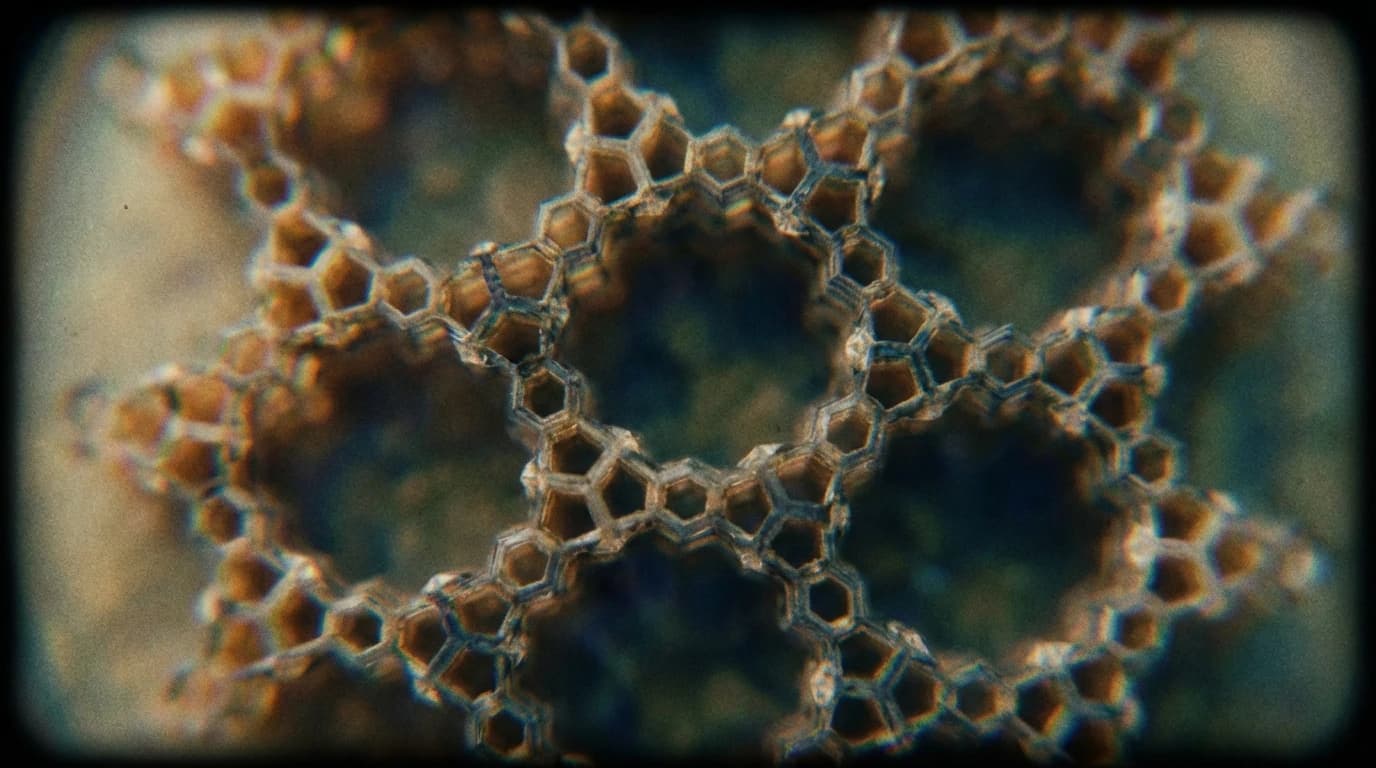
Then comes the structural twist. The solution may not lie in new metals, but in a specific kind of empty space. Covalent organic frameworks (COFs) are entering the scene, offering a level of architectural precision previously absent in battery design.
The Architecture of Efficiency
Unlike the chaotic jumble of traditional electrode materials, COFs are built with intention. They possess a designable topology—a pre-planned grid of atoms that creates ordered pore channels. This review highlights that these channels act as the 'hidden compartments' where the chemistry can finally breathe. By providing a high specific surface area, these frameworks prevent the suffocating buildup of byproducts.
The study indicates that COFs do more than just provide space. They actively participate in the rescue. When used as electrocatalysts, they speed up the sluggish gas-involved reactions that plague Li-O2 and Zn-air batteries. The ordered structure allows ions to move freely, breaking the deadlock of slow kinetics. Furthermore, the chemical stability of these frameworks suggests they can shield the electrolyte from degradation, acting as a sturdy solid-state electrolyte or a protective gas diffusion layer.
Outlook and Challenges
While the laboratory results are compelling, the review notes that real-world application is not imminent. The synthesis of these materials must be scaled, and their long-term durability in fluctuating conditions remains a question mark. However, the path is clear. By replacing random interactions with engineered precision, we may finally stop these next-generation batteries from choking on their own potential.